Stem Cell Research in Age-Related Macular Degeneration
How is stem cell research changing the field of Age-Related Macular Degeneration?
Age-related Macular Degeneration and the CPCB-RPE1 IMplant
What is Age-Related Macular Degeneration?
Age-Related Macular Degeneration, also known as ‘AMD’ is one of the most common causes of poor or worsening vision after the age of 60. This loss of vision is caused by a deterioration or breakdown of the macula, which is a small area in the center of the retina at the back of the eye. The macula is the part of the eye that allows people to see fine details clearly, recognize faces and perform activities such as reading and driving. The symptoms of AMD primarily include loss of central vision, while the peripheral or side vision can remain unaffected.
Although the specific cause of AMD is unknown, some specific risk factors have been identified. While advancing age is the most significant risk factor for developing AMD, having a family history, blue eyes, high blood pressure, cardiovascular disease, and smoking have also been identified as risk factors.
Are there different types of AMD?
There are two types of AMD known as ‘Dry’ or atrophic and ‘Wet’ or exudative.
Exudative or “Wet” AMD is less common (occurring in one out of 10 people with AMD) but is very serious and treatment can be time-sensitive. In the wet form of AMD, abnormal blood vessels may grow in a layer beneath the retina, leaking fluid and blood and creating distortion or a large blind spot in the center of vision. If the blood vessels are not growing directly beneath the macula, laser surgery is usually the treatment of choice. The laser procedure usually does not improve vision but may prevent further vision loss. If the blood vessels are growing directly under the center of the macula, ophthalmologists can provide treatment by injecting anti-vascular endothelial growth factor (anti-VEGF) agents inside the eye, known as an ‘intravitreal injection’. Patients typically receive multiple injections, occurring several times per year. This treatment is beneficial because it inhibits the growth of new blood vessels and shrinks existing leaky vessels. These kinds of injections have been shown to preserve vision for many people and sometimes even restore lost vision for others.
Atrophic or ‘Dry’ AMD is the most common, occurring in 90% of people that have AMD. This form takes many years to develop and results in thinning of the macula. Some patients develop geographic atrophy (GA), which refers to regions of the retina where cells waste away and die (atrophy). GA occurs in 0.34% of people between 65-74 years old, 1.3% between 75-84, 4.4% over 85 years old and increases to 22% after 90 years of age. GA can be diagnosed by an ophthalmologist during an examination after the pupil has been dilated with special eye drops. Special retinal imaging including retinal color photographs, optical coherence tomography (OCT), fluorescein angiography or autofluorescence photographs can also be used to detect and monitor progression of GA. Sometimes these regions of atrophy look like an area on a map to the doctor who is examining the retina, hence the term geographic atrophy. The regions of atrophy result in a blind spot in the visual field. The initial symptom may be noticed when reading, when one or several letters in a word are “missing,” or when looking at faces, a small part of the face cannot be seen. Usually, once GA starts, the region of atrophy expands slowly over several years until the central vision is lost and vision is about 20/200, which means that when this person is 20 feet from an eye chart, they can see what a person with unimpaired (or 20/20) vision can see from 200 feet away. Peripheral vision is not necessarily as affected.
Geographic atrophy (GA) can be present in one or both eyes, and a patient with GA in one eye is more likely to develop it in the other.
Although promising AMD research is being done on many fronts. There is currently no treatment available for GA. Patients can benefit from increased lighting, magnification and low vision devices that help with reading.
In recent years, significant progress has been made in understanding the cause or pathogenesis of GA, which has led to new potential therapies that are currently undergoing evaluation in clinical trials. Studies have shown that the areas of GA that are visible to ophthalmologists during an eye exam are due to cell death in three different areas or layers of the retina. The retina is like layers of wallpaper on the inside, back wall of the eye. Each layer is made of up of different types of cells, each with important roles in maintaining vision. The three areas or layers of the retina that experience cell death in GA are the retinal pigment epithelium or ‘RPE’, the outer neurosensory retina and the choriocapillaris. OCT imaging, noted above, allows ophthalmologists to non-invasively see and assess all of these layers, appearing as brighter and darker bands in an image like a cross-section of the retina.
Regenerative Patch Technologies, LLC (RPT) is a California based company that is developing cell-based implant technology for the treatment of retinal diseases. Their lead product, CPCB-RPE1 is a bioengineered, ultrathin implant covered with a single layer of mature RPE cells and is currently being evaluated in clinical trials. This implant is designed to replace RPE cells that have been lost or damaged in eyes with GA. The implant is carefully delivered to the back of the eye, in the area of GA, in the subretinal space (under a layer of the retinal ‘wallpaper’) where the eyes own cells have been damaged or lost. The procedure is completed as an outpatient day-surgery, under general anesthetic.
RPT recently published the initial results of their first clinical trial using the implant for GA. The trial was designed to assess the safety and effectiveness of the implant. Participants were between 55 and 85 years of age and had experienced severe vision loss due to GA in at least one of their eyes. The initial results demonstrated that over the follow-up period the implant and surgical procedure were safe. Additionally, when these patients underwent eye exams and imaging to assess any structural and functional changes, improvements were observed, suggesting that the CPCB-RPE1 implant may improve visual function in patients with GA. Clinical studies are ongoing and larger clinical trials are being planned.

The CPCB-RPE1 Implant
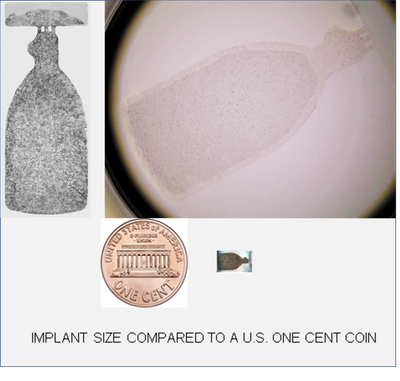
The CPCB-RPE1 Implant
This investigational treatment includes using an implant (CPCB-RPE1) to replace some of the cells in the retina that have been lost or damaged due to Dry AMD.
The implant (CPCB-RPE1) is a small patch with a single layer of cells on a thin membrane which will be surgically inserted between the layers of your retina.
Where do the cells come from?
The RPE cells were produced from human embryonic stem cells which were derived from a single embryo that was intended to be discarded but eventually donated for medical research. These stem cells have been thoroughly tested and approved for such use.
The cells are put onto a very small membrane to make the implant.
* The implant is exclusively for use within the current clinical trial and not available for other experimental indications or compassionate use at this time.
The surgical procedure
The Surgical Implantation Procedure
To deliver the implant to the eye, a vitreo-retinal surgeon will perform a standard small gauge vitrectomy. This is a very common retinal surgery.
A space will be created between the retinal layers where lost or damaged cells were located.
The implant is surgically inserted between the layers of the retina. The small implant has a thin layer of retinal pigment epithelial (RPE) cells. The same type of cells that have been damaged or lost in the eye in geographic atrophy.
*Please note that this is a very brief overview.